Saliva Based Testing Screening Market by Product(Saliva collection kits/devices), Application (Infection analysis/detection) and Global Forecasts, 2023-2032.
Saliva Based Testing Screening Market by Product (Saliva collection kits/devices, Saliva nucleic acid purification kits, Saliva base detection kits, PCR based, Rapid kits), Purpose (Research use only, Diagnostics), Application (Infection analysis/detection, Genomic analysis, Proteomics, Pharmacogenomics, Liquid biopsy), End User (Hospitals & Clinics, Diagnostic laboratories, Academic & research institutes, Biopharmaceuticals & CRO’s) and Geographic Regions (North America, Europe, Asia Pacific, Latin America, Middle East and Africa): Industry Trends and Global Forecasts, 2023-2032.
Market Size and Overview:
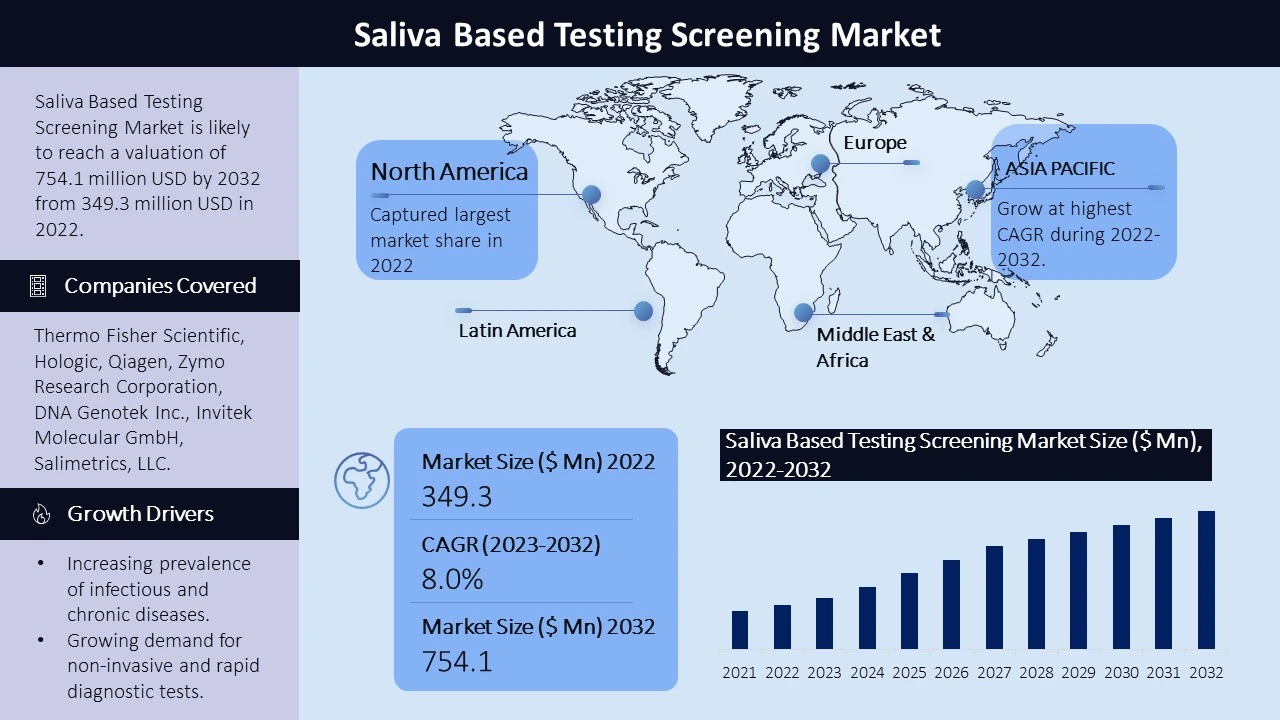
The market for saliva-based testing screening has witnessed significant growth, driven by the rising demand for saliva testing kits and screening. According to the report, the global saliva-based screening market was valued at $349.3 million in 2022 and is projected to reach $754.1 million by the end of 2032, growing at a compound annual growth rate (CAGR) of 8.0% during the forecast period. Saliva-based detection kits are expected to hold the largest market share. The market growth is attributed to the non-invasive nature of saliva collection, the increasing prevalence of diseases, and the growing preference for saliva-based testing over serum-based testing. The market is also driven by innovations in screening technology and the introduction of new kits by manufacturers to meet the growing demand.
Market Segmentation:
Product:
- Saliva collection kits/devices
- Saliva nucleic acid purification kits
- Saliva base detection kits
- PCR based
- Rapid kits
Purpose:
- Research use only
- Diagnostics
Application:
- Infection analysis/detection
- Genomic analysis
- Proteomics
- Pharmacogenomics
- Liquid biopsy
End User:
- Hospitals & Clinics
- Diagnostic laboratories
- Academic & research institutes
- Biopharmaceuticals & CRO’s
Geographic Regions:
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Saliva-based PCR Tests: Saliva-based PCR tests hold the largest market share in the Saliva-Based Testing/Screening market. These tests utilize polymerase chain reaction (PCR) technology to detect the presence of specific genetic material, providing accurate and reliable results. Saliva-based PCR tests are widely used in various healthcare settings due to their convenience, non-invasive nature, and ability to detect infectious diseases effectively.
Hospitals and Clinics: The hospitals and clinics segment represent a significant portion of the market, driven by the need for timely and accurate diagnostic testing. Saliva-based tests are increasingly adopted in these settings due to their ease of use and potential for mass testing. Hospitals and clinics continue to invest in advanced diagnostic technologies, including saliva-based testing, to enhance patient care and improve disease management.
Regional Analysis:
North America region stands as one of the most developed and mature markets for saliva-based testing. The presence of a well-established healthcare infrastructure, high healthcare expenditure, and numerous diagnostic laboratories and hospitals contribute to the market's growth. The COVID-19 pandemic has also fueled the demand for saliva-based tests in this region, leading to increased investment and research in the field.
In Europe, the region showcases a mature market for saliva-based testing. With a robust healthcare infrastructure and a significant number of diagnostic laboratories and hospitals, Europe has been at the forefront of adopting and developing new diagnostic technologies.
In the Asia-Pacific region, rapid population growth and the increasing demand for improved healthcare infrastructure drive the market expansion. The region is witnessing significant investments in diagnostic testing infrastructure and technologies, spurred by the outbreaks of infectious diseases like COVID-19.
Latin America showcases steady growth in the saliva-based testing screening market, driven by urban development, a rising middle class, and a growing demand for healthcare services. Brazil, Mexico, and Argentina emerge as key markets within the region.
the Middle East and Africa exhibit a developing market with a focus on improving healthcare infrastructure, medical tourism, and the promotion of green spaces. The United Arab Emirates, Saudi Arabia, and South Africa are notable contributors to the market's growth in this region.
Growth Drivers:
Adoption of saliva-based testing/screening is growing significantly in the future. Saliva-based testing/screening is an emerging diagnostic technology that offers a convenient, non-invasive, and cost-effective alternative to traditional blood-based tests.
The global saliva-based testing/screening market is being driven by several factors. Firstly, the increasing prevalence of infectious and chronic diseases is fueling the demand for accurate and rapid diagnostic tests. Saliva-based tests provide a non-invasive sampling method that is more comfortable for patients, leading to increased testing rates and early detection of diseases.
Secondly, there is a growing demand for non-invasive and rapid diagnostic tests. Saliva-based tests offer a simple collection process, eliminating the need for needles and reducing patient discomfort. The quick turnaround time for results enables prompt decision-making for healthcare professionals and improves patient care.
Technological advancements in saliva-based testing devices and assays are also contributing to market growth. Innovations in sample collection techniques, detection methods, and test kits are enhancing the accuracy and sensitivity of saliva-based tests. These advancements are driving the adoption of saliva-based testing/screening in various healthcare settings.
Increased investments in research and development by key players and academic institutions are fostering advancements in saliva-based testing/screening technology. These investments are driving the development of innovative products that can detect multiple diseases and conditions simultaneously, expanding the applications and utility of saliva-based tests.
Challenges:
The emergence of new businesses offering nasal swab testing kits, along with challenges such as lack of knowledge, weak diagnostic facilities, and supply of defective products., hampers the growth of the saliva-based testing market.
Key Companies:
The report provides an overview of leading companies in the saliva-based testing screening market, including Thermo Fisher Scientific, Hologic, Qiagen, Zymo Research Corporation, DNA Genotek Inc., Invitek Molecular GmbH, Salimetrics, LLC., BioChain Institute Inc., Mawi DNA Technologies LLC, Cell Projects Ltd., Kyodo International, Inc., Spectrum Solutions, NEOGEN Corporation, Xiamen Zeesan Biotech Co., Ltd., Sedia Biosciences Corporation, Biosynex SA, Chembio Diagnostics, Inc., Creative Diagnostics, OraSure Technologies and other players. These companies are at the forefront of the market, demonstrating strong market presence, extensive distribution networks, and a wide range of product offerings.
To enhance their market share and cater to the diverse needs of customers, these companies employ competitive strategies such as product innovation, strategic partnerships, mergers, and acquisitions. Hologic, Inc. received a CE Mark in July 2021 for the use of saliva samples with the Aptima® SARS-CoV-2 assay in Europe. The Aptima SARS-CoV-2 test is a molecular diagnostic test that identifies the genetic makeup of the COVID-19 infection. This test is performed on the fully automated Panther® system, enabling efficient and accurate detection of the virus in saliva samples.

Need Customized Report for Your Business ?
Utilize the Power of Customized Research Aligned with Your Business Goals
Request for Customized Report- Quick Contact -
- ISO Certified Logo -

















